Evariste is an AI-driven Drug Discovery company
Patient-First Treatment
Evariste identifies and validates novel biomarkers to customize treatment to the patient
AI-Optimized Design
Evariste’s algorithmic modeling designs higher quality, differentiated molecules
Highly Automated R&D
Evariste has automated preclinical drug discovery enabling unprecedented scale and efficiency
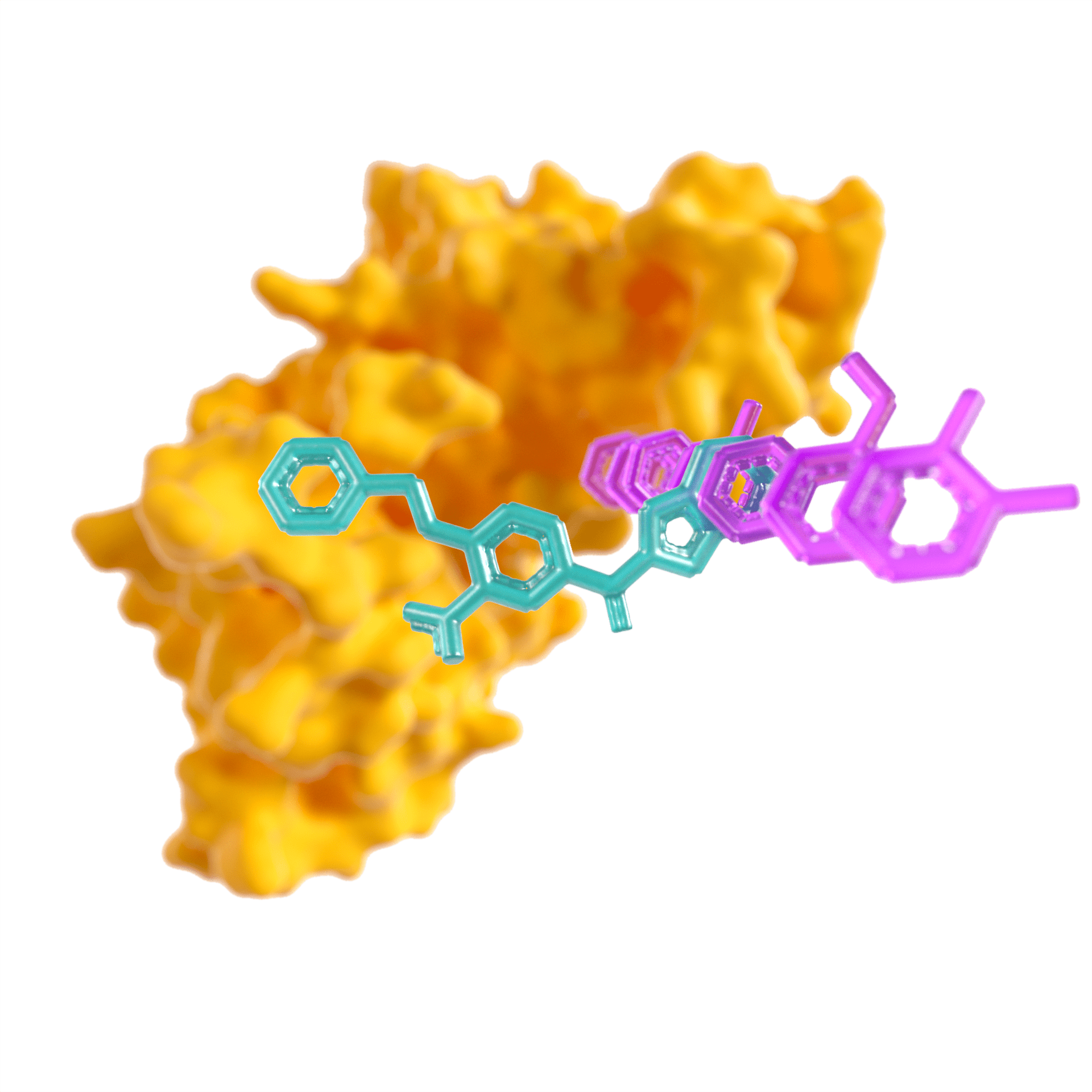
Frobenius is Evariste's AI-enabled small molecule drug discovery platform
Using Frobenius, we are able to de-risk and progress projects with unrivaled efficiency
Each stage of our platform has been validated in challenging internal and collaborative projects
Frobenius Target
At Evariste we have developed the novel concept of synthetic lethal gene signatures, small panels of multi-omic biomarkers which reveal untargeted synthetic lethal interactions together with the patient population for which drugs targeting these genes will be most effective.
Our AI platform applies proprietary algorithms to large multi-omic biological and chemical datasets, including proprietary and public data. Targets are validated in disease-relevant models to ensure their clinical applicability. We identify specific predictive biomarkers or novel synthetic lethalities to ensure that the right patients will receive our treatments.
Frobenius Discovery
To translate the insights derived from Frobenius Target, Frobenius Discovery utilises information-rich hit finding tools to maximise the speed and success of ligand discovery.
We search ultra large libraries for hit-like chemical matter using statistically robust proprietary scoring functions. Probabilistic algorithms exploit the structure of combinatorial libraries, and facilitate virtual screening of libraries consisting of trillions of diverse compounds. The output of our screen is a diverse set of compounds in energetically favorable conformations hyper-enriched in key protein-ligand interactions.
We have successfully applied covalent fragment screens to find novel hit matter for previously undrugged protein families and target classes. The vast quantities of data generated by this technique maximises the speed of downstream development.
Frobenius Candidate
Frobenius Candidate designs and scores synthetically accessible molecules on a scale that would not be feasible for a team of chemists. Probabilistic modeling allows for principled combination of multiple endpoints, facilitating multi-parameter optimization. Our machine learning models are ideally suited to drug discovery as they are designed to work on small, noisy datasets.
The platform stands out not just for its ability to design synthetically accessible molecules, but also for its efficiency and efficacy. Through advanced statistical compound selection techniques, the platform consistently achieves a 10-fold increase in potency for every set of 30 compounds tested. Furthermore, in fewer than 50 compounds it has designed compounds with best-in-class selectivity against targets that are highly homologous. This level of precision and rapid optimization positions the Frobenius platform at the forefront of drug discovery, offering a transformative approach in an era where fast and efficient compound development is paramount.
Using Frobenius, we have developed a pipeline of precision oncology therapeutics
This includes best-in-class inhibitors of validated synthetic lethal targets expanded to new indications, and first-in-class inhibitors of novel targets
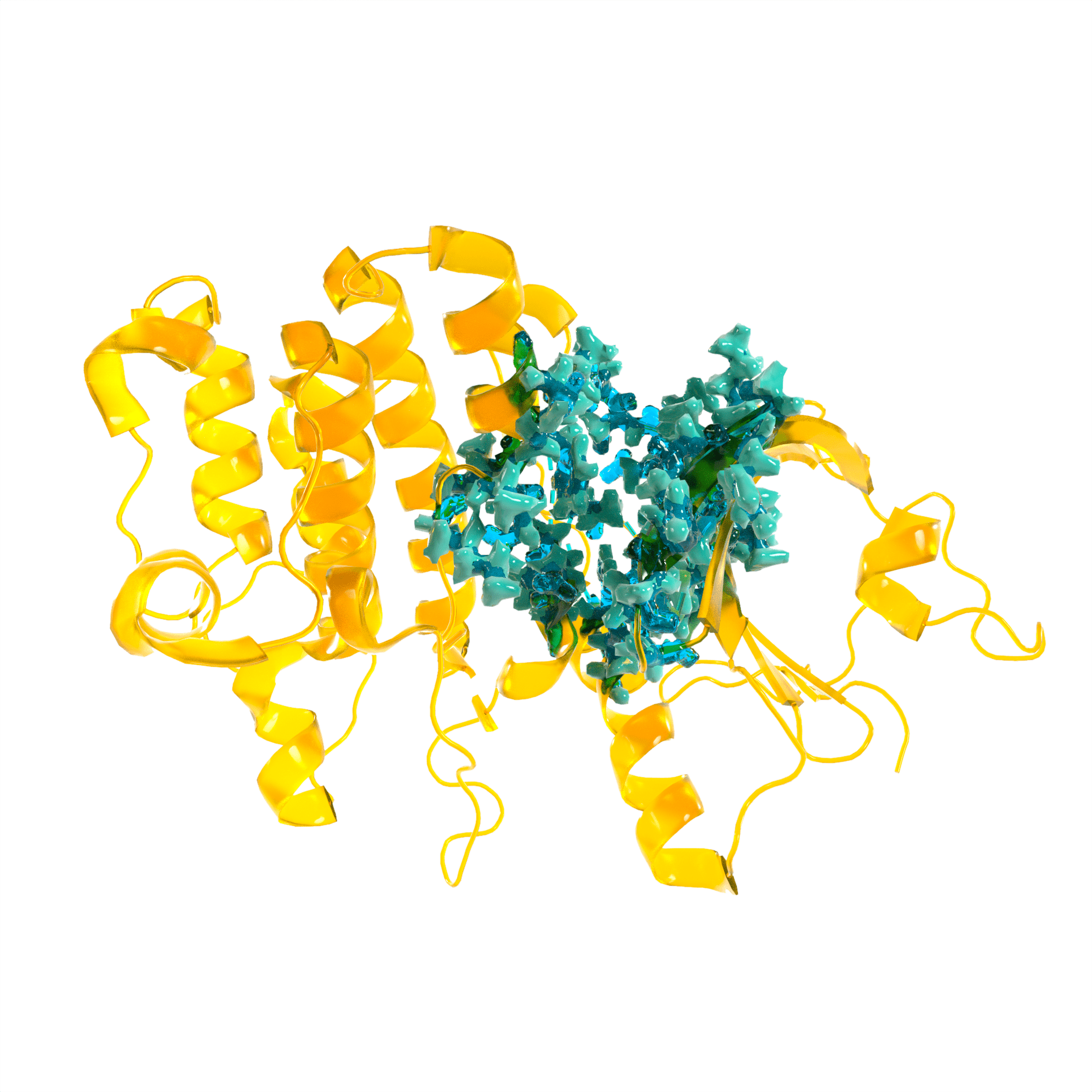
PKMYT1
Targeting PKMYT1 drives synthetic lethality in CCNE1 over-expressing cell lines, with clinical trials ongoing to explore this hypothesis. We have identified a novel biomarker for PKMYT1 synthetic lethality with potential for a significantly expanded patient population, and designed novel inhibitors with high potency and selectivity.
Undisclosed - Haematological Malignancies
Undisclosed target for heavily pretreated hematological malignancies. We have identified a new synthetic lethal target in a subset of acute myeloid leukemia and diffuse large B-cell lymphoma patients. This target is extremely novel and no enzymes of this class have been targeted with small molecule inhibitors or biologics.
Undisclosed - Synthetic Lethal
A well-validated target that is currently in clinical trials without a clear biomarker strategy. We have identified a novel synthetic lethal partner to allow rapid patient selection and have designed inhibitors with best-in-class potency and selectivity.
Discovery
Optimization
Enabling
Team
Mike Waring holds the Chair of Medicinal Chemistry at Newcastle University, he is Head of Chemistry for the Cancer Research UK Newcastle Drug Discovery Unit and Director of the EPSRC Centre for Doctoral Training in Molecular Sciences. He was Principal Scientist in Medicinal Chemistry at AstraZeneca. He is a highly experienced medicinal chemist with a track record of delivering drug-discovery projects through the clinic.

Mike Waring
SCIENTIFIC DIRECTOR
Zoë Walters is a lecturer in translational epigenomics in the school of Cancer Sciences and is a Module Lead on the MSc Genomics within the Faculty of Medicine at Southampton. Zoë is a highly experienced cancer biologist whose expertise lies in target identification and validation in cancer and developmental disorders. Zoë has 18+ years' experience in molecular genetics, developmental biology, and cancer biology.

Zoë Walters
SCIENTIFIC DIRECTOR
Daniel received a PhD in Cell and Molecular Biology from the Francis Crick Institute, studying the signalling dynamics of the TGF-β family, before working with AstraZeneca to develop therapeutic antibodies. He then worked at the Institute of Cancer Research, where his work focused on understanding the mechanism of action of molecular glue-like small molecules.

Daniel Miller
PRINCIPAL SCIENTIST BIOLOGY
Jan received a PhD in Medicinal Chemistry from the University of Southern Denmark, working on AMPK modulators. He then joined the Institute of Cancer Research, optimizing inhibitors of a kinesin motor protein and subsequently worked on the development of antibody-drug conjugates at AstraZeneca.

Jan Lanz
PRINCIPAL SCIENTIST CHEMISTRY
Partnerships
We work in collaboration with biotech companies to accelerate the development of small molecules for a range of target classes in diverse disease areas. Our AI platform is able to support and advance discovery at every stage of preclinical development.
For any collaboration or business development inquiries:
Our Partners


Contact us
News & updates
Stay updated with Evariste's blogposts